DanCann Pharma A/S: Pioneers in cannabinoids
DanCann Pharma A/S is a Danish biopharmaceutical company powered by cannabinoids, which comprises a consortium of companies dedicated to the commercial aspects of the EU-GxP sphere.
This includes a focus on market access, sourcing, and distribution, with a specialised emphasis on cannabinoid medicines and medications targeting the endocannabinoid system, and novel treatment modalities.
The portfolio and pipeline encompass both generic and proprietary IP products, with the aim of ensuring a comprehensive and extensive product portfolio.

DanCann Pharma A/S: Pioneers in cannabinoids
DanCann Pharma A/S is a Danish biopharmaceutical company powered by cannabinoids, which comprises a consortium of companies dedicated to the commercial aspects of the EU-GxP sphere.
This includes a focus on market access, sourcing, and distribution, with a specialised emphasis on cannabinoid medicines and medications targeting the endocannabinoid system, and novel treatment modalities.
The portfolio and pipeline encompass both generic and proprietary IP products, with the aim of ensuring a comprehensive and extensive product portfolio.
The DCP foundation:
Mission, vision, and values statements are the foundation for our strategic plan. They state the purpose, direction, and underlying values of DanCann Pharma while serving as a tool that provides us with guidance and direction.
- General
To supply relief to those given up on by the healthcare system. Condition; Unmet needs.
Our dream is about giving new hope to patients and their families who are left behind by the conventional healthcare industry, and to inspire a movement towards a better future for all.
2018
The emergence of medicinal cannabis and the birth of DanCann.
CannGros ApS - your medicinal cannabis supplier
DanCann Pharma conducts its Danish operations through its subsidiary, known as CannGros ApS, which possesses the necessary licenses for handling narcotic substances, as well as importing and distributing cannabis primary- and intermediate products respectively.
CannGros manages the importation of medicinal products and narcotics while also overseeing primary and secondary packaging, warehousing, logistics, shipping, labeling, and batch release.
CannGros maintains partnerships with the major wholesalers in Denmark, facilitating the swift delivery of its portfolio to all pharmacies across the nation.
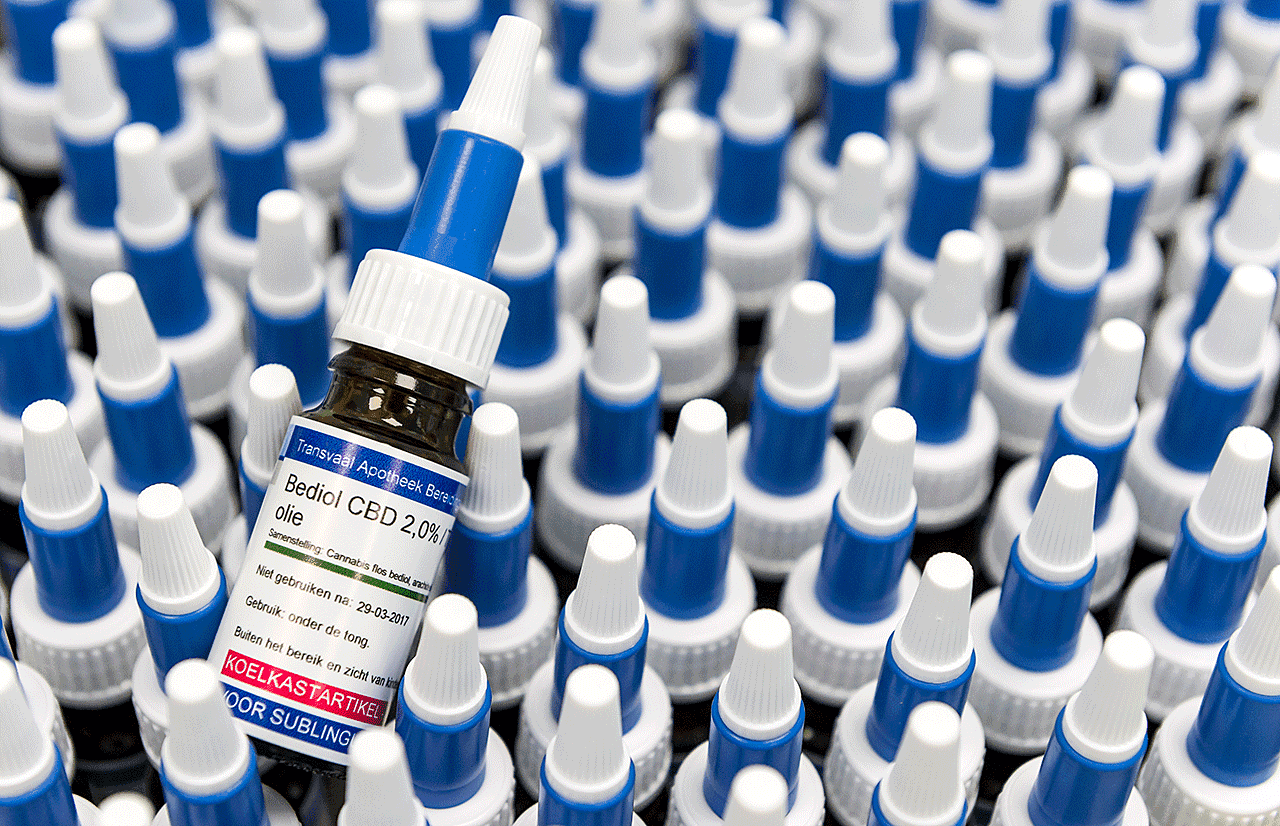
CannGros ApS - your medicinal cannabis supplier
DanCann Pharma conducts its Danish operations through its subsidiary, known as CannGros ApS, which possesses the necessary licenses for handling narcotic substances, as well as importing and distributing cannabis primary- and intermediate products respectively.
CannGros manages the importation of medicinal products and narcotics while also overseeing primary and secondary packaging, warehousing, logistics, shipping, labeling, and batch release.
CannGros maintains partnerships with the major wholesalers in Denmark, facilitating the swift delivery of its portfolio to all pharmacies across the nation.
Associated member of:
Join the cannabinoid revolution today
We are always on the lookout for our next teammate and go through applications every day. If you find an opportunity that speaks to you, please apply and send us your resume with a few sentences describing why you believe we are a great match.


